FDA Drug Safety Communication: FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes (2015)
The U.S. Food and Drug Administration (FDA) is strengthening an existing label warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the chance of a heart attack or stroke. Based on our comprehensive review of new safety information, we are requiring updates to the drug labels of all prescription NSAIDs. As is the case with current prescription NSAID labels, the Drug Facts labels of over-the-counter (OTC) non-aspirin NSAIDs already contain information on heart attack and stroke risk. We will also request updates to the OTC non-aspirin NSAID Drug Facts labels.
Patients taking NSAIDs should seek medical attention immediately if they experience symptoms such as chest pain, shortness of breath or trouble breathing, weakness in one part or side of their body, or slurred speech.
NSAIDs are widely used to treat pain and fever from many different long- and short-term medical conditions such as arthritis, menstrual cramps, headaches, colds, and the flu. NSAIDs are available by prescription and OTC. Examples of NSAIDs include ibuprofen, naproxen, diclofenac, and celecoxib (see Table 1 for a list of NSAIDs).
The risk of heart attack and stroke with NSAIDs, either of which can lead to death, was first described in 2005 in the Boxed Warning and Warnings and Precautions sections of the prescription drug labels. Since then, we have reviewed a variety of new safety information on prescription and OTC NSAIDs, including observational studies,1 a large combined analysis of clinical trials,2 and other scientific publications.1 These studies were also discussed at a joint meeting of the Arthritis Advisory Committee and Drug Safety and Risk Management Advisory Committee held on February 10-11, 2014.
Based on our review and the advisory committees’ recommendations, the prescription NSAID labels will be revised to reflect the following information:
- The risk of heart attack or stroke can occur as early as the first weeks of using an NSAID. The risk may increase with longer use of the NSAID.
- The risk appears greater at higher doses.
- It was previously thought that all NSAIDs may have a similar risk. Newer information makes it less clear that the risk for heart attack or stroke is similar for all NSAIDs; however, this newer information is not sufficient for us to determine that the risk of any particular NSAID is definitely higher or lower than that of any other particular NSAID.
- NSAIDs can increase the risk of heart attack or stroke in patients with or without heart disease or risk factors for heart disease. A large number of studies support this finding, with varying estimates of how much the risk is increased, depending on the drugs and the doses studied.
- In general, patients with heart disease or risk factors for it have a greater likelihood of heart attack or stroke following NSAID use than patients without these risk factors because they have a higher risk at baseline.
- Patients treated with NSAIDs following a first heart attack were more likely to die in the first year after the heart attack compared to patients who were not treated with NSAIDs after their first heart attack.
- There is an increased risk of heart failure with NSAID use.
We will request similar updates to the existing heart attack and stroke risk information in the Drug Facts labels of OTC non-aspirin NSAIDs.
In addition, the format and language contained throughout the labels of prescription NSAIDs will be updated to reflect the newest information available about the NSAID class.
Patients and health care professionals should remain alert for heart-related side effects the entire time that NSAIDs are being taken. We urge you to report side effects involving NSAIDs to the FDA MedWatch program, using the information in the “Contact FDA” box at the bottom of the page
Hola, Este Tema lo habíamos revisado hace un año.
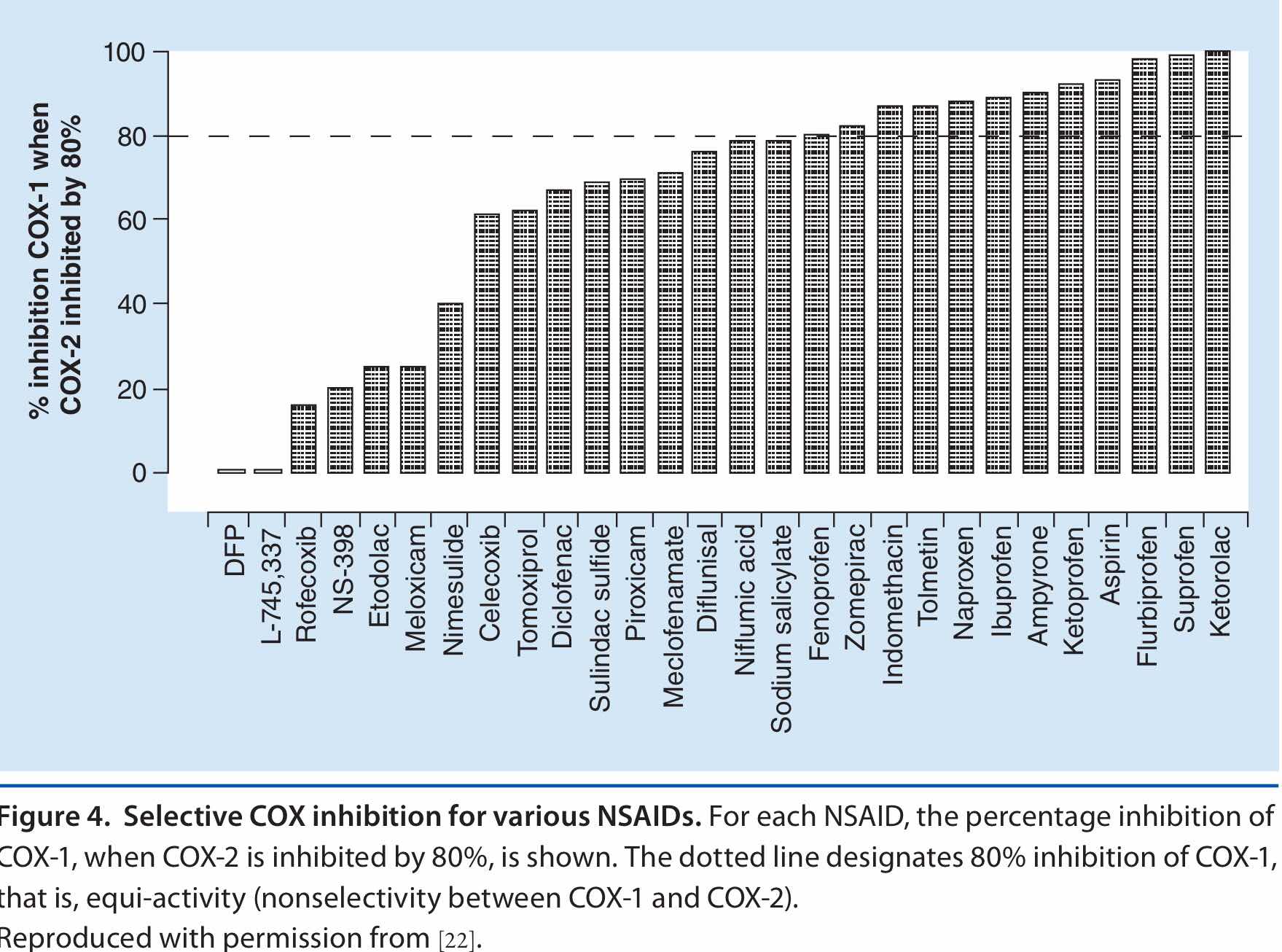
Este es el comunicado emitido por la FDA en el 2015 (No era el primero) que buscaba socializar la preocupación de la comunidad médica, acerca de la toxicidad cardiovascular de los AINES. Esta toxicidad se presenta con todos los AINES sin excepción, sin embargo su magnitud varía en forma importante según el medicamento específico y no su familia.
El diclofenaco es uno de los AINES más utilizados y como todos sus congéneres produce cardiotoxicidad, sin embargo, diferentes estudios han demostrado que su nivel de cardiotoxicidad puede ser comparado con el de los COX-2 selectivos como el Rofecoxib (retirado del mercado)
1. Cual es el mecanismo por el cual un AINE produce cardiotoxicidad.
2. Por que razón el diclofenaco es, según algunos estudios, mas cardiotóxico .
3. Que población es más susceptible. En quienes se puede utilizar ?
4. Que estrategia de manejo se recomienda en pacientes de alto riesgo ?
5. Se modifica este riesgo cuando se administran por otra via (IM, IV, transdérmico) ?
6. Cual es el perfil de seguridad del PARECOXIB - Que estudios soportan su uso.
Nos vemos